Tetra Images/Getty Images
- The FDA has approved KemPharm’s once-daily treatment for ADHD, AZSTARYS.
- The prodrug was praised by Dr. Ann Childress, President of the Center for Psychiatry and Behavioral Medicine.
- KemPharm is eligible to receive up to $468 million in milestone payments as well as royalties from sales of the prodrug.
- Watch KemPharm trade live here.
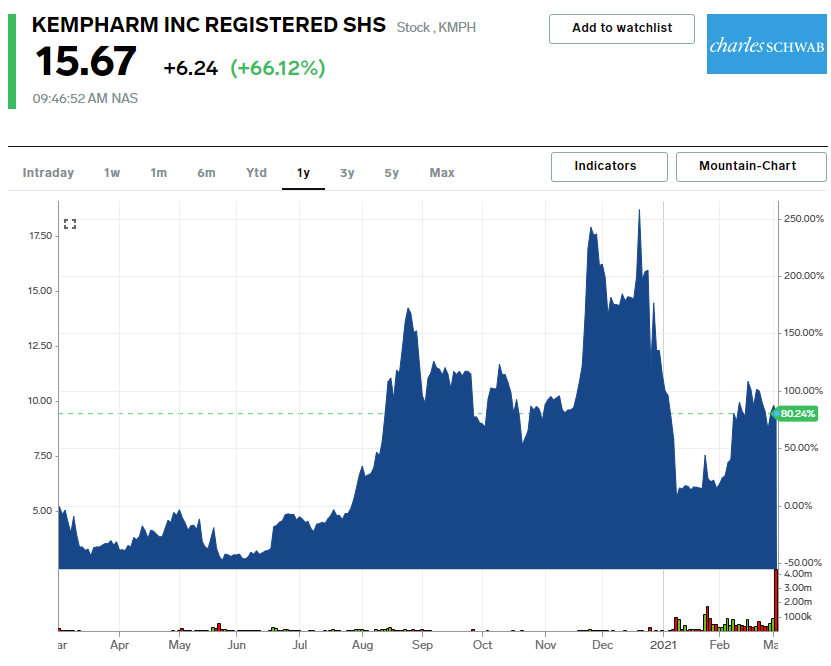
Shares of KemPharm skyrocketed as much as 90% on Wednesday after the FDA approved the company’s once-daily treatment for ADHD called Azstarys.
The FDA’s approval of Azstarys, will earn KemPharm a regulatory milestone payment due to the company’s licensing agreement with the commercial-stage biopharmaceutical company Corium.
Under the License Agreement, KemPharm will be eligible for up to $468 million in regulatory and sales milestone payments, as well as significant tiered royalty payments.
KemPharm uses Ligand Activated Therapy (LAT) to discover and develop prodrugs which are used to improve a drug’s bioavailability, extend its duration of action, reduce its susceptibility to abuse, and/or increase safety.
KemPharm’s stock was down nearly 15% over the past six months before Wednesday’s jump in share prices. The company hit highs of $18.72 per share on December 18 of last year and has seen its stock fall since, but the recent FDA approval was a game-changer for the biotech firm, accord to the company’s CEO.
"The FDA approval of the Azstarys NDA is a transformational event for KemPharm and, we believe, an important advancement in the treatment of ADHD," said Travis C. Mickle, president and CEO of KemPharm.
"Today's approval highlights both the value potential of SDX, our prodrug of d-MPH, and the ability of our LAT platform technology to develop new prodrugs of approved medications that improve one or more of the attributes of the parent drug," the CEO added.
Although prodrugs aren't well known, an estimated 10% of drugs worldwide are considered to be prodrugs.
The Centers for Disease Control and Prevention estimates that 8.4% of children and 2.5% of adults in the US have ADHD. And according to data from Grandview Research, the ADHD pharmacology market could hit $24.9 billion by 2025.
Ann Childress, M.D. and President of the Center for Psychiatry and Behavioral Medicine, noted the positive results of the Azstarys clinical trial in a statement. Childress said:
"The ADHD industry, and specifically the MPH space, has seen little innovation in recent years, leaving prescribers and patients desiring new treatment options. In my research and practice, three properties are repeatedly cited by patients and their caregivers as being underserved by current ADHD medications: onset of action, duration of effect and consistency of therapy. Having investigated AZSTARYS and directly observed its clinical impact on patients, I believe this product will be an important new tool for physicians to use in providing effective care for patients with ADHD."